Difference between revisions of "User:Tohline/Appendix/Equation templates"
(→[[Image:LSU_Key.png|50px]]<font size="+2" color="darkblue">Key Equations</font>: split equation table into several parts) |
|||
| (141 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
__FORCETOC__ | |||
<!-- __NOTOC__ will force TOC off --> | |||
=[[Image:LSU_Key.png|50px]]<font size="+2" color="darkblue">Key Equations</font>= | |||
{{LSU_HBook_header}} | {{LSU_HBook_header}} | ||
Each of the equations displayed in the Tables, below, encapsulates a physical concept that is fundamental to our understanding of — and, hence our discussion of — the '''structure, stability, and dynamics of self-gravitating fluids.''' The pervasiveness of these physical concepts throughout astrophysics is reflected in the fact that the same equations — perhaps written in slightly different forms — appear in numerous published books and research papers. When attempting to understand the physical concept that is associated with any one of these mathematical relations, it can be helpful to read how and in what context different authors have introduced the expression in their own work. These Tables offer guides to some parallel discussions that have appeared in published texts over the past 5+ decades in connection with selected sets of key physical relations. | |||
Each of the equations displayed in the | |||
<font color="darkgreen">EXAMPLE:</font> Suppose you want to gain a better understanding of the origin of the ideal gas equation of state, the definition of the gas constant {{User:Tohline/Math/C_GasConstant}}, or how to determine the value of the mean molecular weight {{User:Tohline/Math/MP_MeanMolecularWeight}} of a gas. According to the Table entitled ''Equations of State'', you will find a discussion of the ideal gas equation of state: near Eq. (1) in §II.1 of Chandrasekhar (1967); near Eq. (80.8) in §IX.80 of Landau & Lifshitz (1975); near Eq. (5.91) in Vol. I, §5.6 of Padmanabhan (2000); etc. A "note" (linked to a comment further down on this page) appears along with a table entry if the relevant equation in the cited reference contains notations or symbol names that differ significantly from the equation as displayed here. | |||
==Principal Governing Equations== | |||
<span id="PGE"> | |||
<div align="center"> | <div align="center"> | ||
<table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | <table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | ||
<tr> | <tr> | ||
<th colspan= | <th colspan=9 align="center"> | ||
<font size="+1" color="darkblue">Principal Governing Equations</font> | <font size="+1" color="darkblue">Principal Governing Equations</font> | ||
</th> | </th> | ||
| Line 18: | Line 23: | ||
{{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | {{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | ||
</td> | </td> | ||
<td colspan= | <td colspan=7 align="center"> | ||
<font color="red">Parallel References</font> <br />§ no. and (Eq. no.) | <font color="red">Parallel References</font> <br />§ no. and (Eq. no.) | ||
</td> | </td> | ||
| Line 24: | Line 29: | ||
<tr> | <tr> | ||
<th width=" | <th width="15%"> | ||
<font color="red">Template_Name</font> | <font color="red">Template_Name</font> | ||
</th> | </th> | ||
| Line 40: | Line 45: | ||
</th> | </th> | ||
<th colspan=1> | <th colspan=1> | ||
<font color="red"> | <font color="red">ST83</font> | ||
</th> | </th> | ||
<th colspan=1> | <th colspan=1> | ||
| Line 47: | Line 52: | ||
<th colspan=1> | <th colspan=1> | ||
<font color="red">P00</font> | <font color="red">P00</font> | ||
</th> | |||
<th colspan=1> | |||
<font color="red">BLRY07</font> | |||
</th> | </th> | ||
</tr> | </tr> | ||
| Line 53: | Line 61: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_Continuity01|EQ_Continuity01]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 63: | Line 71: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§I.1<br /> (1.2)<br />[ | §I.1<br /> (1.2)<br />[[#LL75note_Continuity01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§5.4<br /> (5.37)<br />[ | §5.4<br /> (5.37)<br />[[#H87note_Continuity01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
& | §6.1<br /> (6.1.1)<br />[[#ST83note_Continuity01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§2.5<br /> (2.22)<br />[ | §2.5<br /> (2.22)<br />[[#KW94note_Continuity01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
I: §8.5<br /> (8.45) | I: §8.5<br /> (8.45) | ||
</td> | |||
<td colspan=1 align="center"> | |||
§1.4<br /> (1.53) | |||
</td> | </td> | ||
</tr> | </tr> | ||
| Line 82: | Line 93: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_Euler01|EQ_Euler01]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 92: | Line 103: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§I.2<br /> (2.1)<br />[ | §I.2<br /> (2.1)<br />[[#LL75note_Euler01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§5.4<br /> (5.38)<br />[ | §5.4<br /> (5.38)<br />[[#H87note_Euler01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
& | §6.1<br /> (6.1.2)<br /> | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| Line 105: | Line 116: | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
I: §8.5<br /> (8.48) | I: §8.5<br /> (8.48) | ||
</td> | |||
<td colspan=1 align="center"> | |||
§1.4<br /> (1.55) | |||
</td> | </td> | ||
</tr> | </tr> | ||
| Line 111: | Line 125: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_FirstLaw01|EQ_FirstLaw01]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 121: | Line 135: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§I.2<br /> (2.5)<br />[ | §I.2<br /> (2.5)<br />[[#LL75note_FirstLaw01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§4.2<br /> (4.31)<br />[ | §4.2<br /> (4.31)<br />[[#H87note_FirstLaw01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
& | §6.1<br /> (6.1.8)<br /> | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§4.1<br /> (4.1)<br />[ | §4.1<br /> (4.1)<br />[[#KW94note_FirstLaw01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
I: §8.5<br /> (8.53) | I: §8.5<br /> (8.53) | ||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | </td> | ||
</tr> | </tr> | ||
| Line 140: | Line 157: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_Poisson01|EQ_Poisson01]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 150: | Line 167: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§I.3<br /> (3.5)<br />[ | §I.3<br /> (3.5)<br />[[#LL75note_Poisson01|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| Line 156: | Line 173: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
& | §6.1<br /> (6.1.4)<br /> | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| Line 162: | Line 179: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
I: §10.2<br /> (10.1)<br />[ | I: §10.2<br /> (10.1)<br />[[#P00note_Poisson01|Note]] | ||
</td> | |||
<td colspan=1 align="center"> | |||
Chap. 7 | |||
</td> | </td> | ||
</tr> | </tr> | ||
| Line 168: | Line 188: | ||
</table> | </table> | ||
</div> | </div> | ||
</span> | |||
==Equations of State== | |||
< | <span id="EOS"> | ||
<div align="center"> | <div align="center"> | ||
<table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | <table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | ||
<tr> | <tr> | ||
<th colspan= | <th colspan=9 align="center"> | ||
<font size="+1" color="darkblue">Equations of State</font> | <font size="+1" color="darkblue">Equations of State</font> | ||
</th> | </th> | ||
| Line 185: | Line 204: | ||
{{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | {{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | ||
</td> | </td> | ||
<td colspan= | <td colspan=7 align="center"> | ||
<font color="red">Parallel References</font> <br />§ no. and (Eq. no.) | <font color="red">Parallel References</font> <br />§ no. and (Eq. no.) | ||
</td> | </td> | ||
| Line 191: | Line 210: | ||
<tr> | <tr> | ||
<th width=" | <th width="15%"> | ||
<font color="red">Template_Name</font> | <font color="red">Template_Name</font> | ||
</th> | </th> | ||
| Line 207: | Line 226: | ||
</th> | </th> | ||
<th colspan=1> | <th colspan=1> | ||
<font color="red"> | <font color="red">ST83</font> | ||
</th> | </th> | ||
<th colspan=1> | <th colspan=1> | ||
| Line 214: | Line 233: | ||
<th colspan=1> | <th colspan=1> | ||
<font color="red">P00</font> | <font color="red">P00</font> | ||
</th> | |||
<th colspan=1> | |||
<font color="red">BLRY07</font> | |||
</th> | </th> | ||
</tr> | </tr> | ||
| Line 220: | Line 242: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_EOSideal0A|EQ_EOSideal0A]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 227: | Line 249: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§II.1<br /> (1) <br />[ | §II.1<br /> (1) <br />[[#C67note_EOSideal0A|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§IX.80<br /> (80.8) <br />[ | §IX.80<br /> (80.8) <br />[[#LL75note_EOSideal0A|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| Line 236: | Line 258: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
& | §2.3<br /> (2.3.32)<br /> or <br /> (3.2.12) | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§13.0<br /> (13.1) <br />[ | §13.0<br /> (13.1) <br />[[#KW94note_EOSideal0A|Note]] | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
I: §5.6<br /> (5.91) | I: §5.6<br /> (5.91) | ||
</td> | |||
<td colspan=1 align="center"> | |||
§5.4<br /> (5.34) | |||
</td> | </td> | ||
</tr> | </tr> | ||
| Line 249: | Line 274: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_ZTFG01|EQ_ZTFG01]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
Degenerate Electron Pressure:<br /> | Degenerate Electron Pressure:<br /> | ||
{{User:Tohline/Math/EQ_ZTFG01}} | {{User:Tohline/Math/EQ_ZTFG01}} | ||
——— <font color="darkgreen">NOTE:</font> ———<br /> | |||
<math> | |||
F(\chi) = \frac{8}{5}\chi^5 - \frac{4}{7}\chi^7 + \cdots ~~~~~~(\mathrm{for}~~ \chi\ll 1) | |||
</math> | |||
<math> | |||
F(\chi) = 2\chi^4 - 2\chi^2 + \cdots ~~~~~~~(\mathrm{for}~~ \chi\gg 1) | |||
</math> | |||
</td> | |||
<td colspan=1 align="center"> | |||
§X.1<br /> (19) <br /> + <br /> (20) | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
§11.2<br /> (11.41) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§2.3<br /> (2.3.5)<br /> + <br /> (2.3.6) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§15.0<br /> (15.13)<br /> + <br /> (15.14) | |||
</td> | |||
<td colspan=1 align="center"> | |||
I: §5.9.2<br /> (5.156)<br /> + <br /> (5.158) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§5.6.1<br /> (5.86)<br /> + <br /> (5.87)<br /> + <br /> (5.88) | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_EOSradiation01|EQ_EOSradiation01]] | |||
</td> | |||
<td align="center"> | |||
Radiation Pressure:<br /> | |||
{{User:Tohline/Math/EQ_EOSradiation01}} | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
§12.1<br /> (12.12)<br /> +<br /> (12.15) | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
§5.6.1<br /> (5.85) | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_PressureTotal01|EQ_PressureTotal01]] | |||
</td> | |||
<td align="center"> | |||
Normalized Total Pressure:<br /> | |||
{{User:Tohline/Math/EQ_PressureTotal01}} | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| Line 262: | Line 370: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§ | | ||
</td> | |||
</tr> | |||
</table> | |||
</div> | |||
</span> | |||
==Traditional Equations of (Spherical) Stellar Structure== | |||
<span id="SS"> | |||
<div align="center"> | |||
<table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | |||
<tr> | |||
<th colspan=9 align="center"> | |||
<font size="+1" color="darkblue">Traditional Equations of (Spherical) Stellar Structure</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td colspan=2> | |||
To insert a given equation into any Wiki document, type ...<br /><center> | |||
{{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | |||
</td> | |||
<td colspan=7 align="center"> | |||
<font color="red">Parallel References</font> <br />§ no. and (Eq. no.) | |||
</td> | |||
</tr> | |||
<tr> | |||
<th width="15%"> | |||
<font color="red">Template_Name</font> | |||
</th> | |||
<th width="40%"> | |||
<font color="red">Resulting Equation</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">C67</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">LL75</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">H87</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">ST83</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">KW94</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">P00</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">BLRY07</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_SSmassConservation01|EQ_SSmassConservation01]] | |||
</td> | |||
<td align="center"> | |||
Mass Conservation:<br /> | |||
{{User:Tohline/Math/EQ_SSmassConservation01}} | |||
</td> | |||
<td colspan=1 align="center"> | |||
§IV.2<br /> (6) | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
§3.2<br /> (3.2.1) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§2.1<br /> (2.4) | |||
</td> | |||
<td colspan=1 align="center"> | |||
II: §2.2<br /> (2.2) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§5.1<br /> (5.2) | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_SShydrostaticBalance01|EQ_SShydrostaticBalance01]] | |||
</td> | |||
<td align="center"> | |||
Hydrostatic Balance:<br /> | |||
{{User:Tohline/Math/EQ_SShydrostaticBalance01}} | |||
</td> | |||
<td colspan=1 align="center"> | |||
§IV.2<br /> (6) | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
§3.2<br /> (3.2.2) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§1.1<br /> (1.2) <br />[[#KW94note_SShydrostaticBalance|Note]] | |||
</td> | |||
<td colspan=1 align="center"> | |||
II: §2.2<br /> (2.1) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§5.1<br /> (5.1) | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_SSLaneEmden01|EQ_SSLaneEmden01]] | |||
</td> | |||
<td align="center"> | |||
Polytropic Lane-Emden Equation:<br /> | |||
{{User:Tohline/Math/EQ_SSLaneEmden01}}<br /> | |||
[[File:EmdenGaskugeln1907.jpg|200px|thumb|left|Emden (1907)]] [[File:RitterEquation1880.jpg|90px|thumb|right|Ritter (1880)]] | |||
</td> | |||
<td colspan=1 align="center"> | |||
§IV.2<br /> (11) <br />[[#C67note_SSLaneEmden01|Note]] | |||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| Line 269: | Line 510: | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| | ||
</td> | |||
<td colspan=1 align="center"> | |||
§3.3<br /> (3.3.6) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§19.2<br /> (19.10) | |||
</td> | |||
<td colspan=1 align="center"> | |||
I: §10.3<br /> (10.4) | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_SSLaneEmden02|EQ_SSLaneEmden02]] | |||
</td> | |||
<td align="center"> | |||
Isothermal Lane-Emden Equation:<br /> | |||
{{User:Tohline/Math/EQ_SSLaneEmden02}}<br /> | |||
</td> | |||
<td colspan=1 align="center"> | |||
§IV.22<br /> (374) <br /> | |||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| | ||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
§19.8<br /> (19.35) | |||
</td> | |||
<td colspan=1 align="center"> | |||
I: §10.3.3<br /> (10.23) | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | </td> | ||
</tr> | </tr> | ||
| Line 278: | Line 560: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_SSradiationTransport01|EQ_SSradiationTransport01]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
Radiation | Radiation Transport:<br /> | ||
{{User:Tohline/Math/ | {{User:Tohline/Math/EQ_SSradiationTransport01}}<br /> | ||
</td> | |||
<td colspan=1 align="center"> | |||
§IV.22<br /> (374) <br /> | |||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| Line 291: | Line 576: | ||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
§ | | ||
</td> | |||
<td colspan=1 align="center"> | |||
§5.1.2<br /> (5.11) | |||
</td> | |||
<td colspan=1 align="center"> | |||
II: §2.2<br /> (2.8) | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_SSenergyConservation01|EQ_SSenergyConservation01]] | |||
</td> | |||
<td align="center"> | |||
Energy Conservation:<br /> | |||
{{User:Tohline/Math/EQ_SSenergyConservation01}}<br /> | |||
</td> | |||
<td colspan=1 align="center"> | |||
§IV.22<br /> (374) <br /> | |||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| Line 298: | Line 606: | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| | ||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
§4.2<br /> (4.22) | |||
</td> | |||
<td colspan=1 align="center"> | |||
II: §2.2<br /> (2.18) | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
</tr> | |||
</table> | |||
</div> | |||
</span> | |||
<br /> | |||
==Stability: Radial Pulsation== | |||
<span id="RadialStability"> | |||
<div align="center"> | |||
<table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | |||
<tr> | |||
<th colspan="7" align="center"> | |||
<font size="+1" color="darkblue">Stability: Radial Pulsation</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td colspan=2> | |||
To insert a given equation into any Wiki document, type ...<br /><center> | |||
{{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | |||
</td> | |||
<td colspan=5 align="center"> | |||
<font color="red">Parallel References</font> <br />§ no. and (Eq. no.) | |||
</td> | |||
</tr> | |||
<tr> | |||
<th width="15%"> | |||
<font color="red">Template_Name</font> | |||
</th> | |||
<th width="40%"> | |||
<font color="red">Resulting Equation</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">C67</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">ST83</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">KW94</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">HK94</font> | |||
</th> | |||
<th colspan=1> | |||
<font color="red">P00</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_RadialPulsation01|EQ_RadialPulsation01]] | |||
</td> | |||
<td align="center"> | |||
LAWE: Linear Adiabatic Wave (or Radial Pulsation) Equation<br /> | |||
{{User:Tohline/Math/EQ_RadialPulsation01}}<br /> | |||
[[File:Eddington1930Cover.png|100px|thumb|right|Eddington (1926)]] | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
§6.5<br /> (6.5.6) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§38.1<br /> (38.8) | |||
</td> | |||
<td colspan=1 align="center"> | |||
§10.1.1<br /> (10.16) | |||
</td> | |||
<td colspan=1 align="center"> | |||
II: §3.7.1<br /> (3.144) | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_RadialPulsation02|EQ_RadialPulsation02]] | |||
</td> | |||
<td align="center"> | |||
Polytropic LAWE:<br /> | |||
{{User:Tohline/Math/EQ_RadialPulsation02}}<br /> | |||
</td> | </td> | ||
<td colspan=1 align="center"> | <td colspan=1 align="center"> | ||
| | ||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_RadialPulsation03|EQ_RadialPulsation03]] | |||
</td> | |||
<td align="center"> | |||
Isothermal LAWE:<br /> | |||
{{User:Tohline/Math/EQ_RadialPulsation03}}<br /> | |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
<td colspan=1 align="center"> | |||
| |||
</td> | |||
</tr> | |||
</table> | </table> | ||
</div> | </div> | ||
</span> | |||
<br /> | <br /> | ||
==<font size="+ | ==Special Function Relationships== | ||
===Gamma Function=== | |||
<div align="center"> | |||
<table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | |||
<tr> | |||
<th colspan=3 align="center"> | |||
<font size="+1" color="darkblue">Gamma Function</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td colspan=2> | |||
To insert a given equation into any Wiki document, type ...<br /><center> | |||
{{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | |||
</td> | |||
<td colspan=1 rowspan="2" align="center"> | |||
<br /> <br /> <br /> | |||
<font color="red">See also …</font> | |||
</td> | |||
</tr> | |||
<tr> | |||
<th width="10%"> | |||
<font color="red">Template_Name</font> | |||
</th> | |||
<th width="75%"> | |||
<font color="red">Resulting Equation</font> | |||
</th> | |||
</tr> | |||
* [<b><font color="red">C67</font></b>] '''Chandrasekhar, S.''' 1967 (originally, 1939), An Introduction to the Study of Stellar Structure (New York: Dover) | <tr> | ||
<td> | |||
[[User:Tohline/Math/EQ_Gamma01|EQ_Gamma01]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Gamma01}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
*[https://authors.library.caltech.edu/43491/1/Volume%201.pdf A. Erdélyi (1953)]: Volume I, §1.2, p. 3, eq. (6) | |||
* [https://en.wikipedia.org/wiki/Gamma_function#General Wikipedia] | |||
</td> | |||
</tr> | |||
</table> | |||
</div> | |||
===Complete Elliptic Integrals=== | |||
<div align="center"> | |||
<table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | |||
<tr> | |||
<th colspan=3 align="center"> | |||
<font size="+1" color="darkblue">Complete Elliptic Integral …</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td colspan=2> | |||
To insert a given equation into any Wiki document, type ...<br /><center> | |||
{{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | |||
</td> | |||
<td colspan=1 rowspan="2" align="center"> | |||
<br /> <br /> <br /> | |||
<font color="red">See also …</font> | |||
</td> | |||
</tr> | |||
<tr> | |||
<th width="10%"> | |||
<font color="red">Template_Name</font> | |||
</th> | |||
<th width="75%"> | |||
<font color="red">Resulting Equation</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_EllipticIntegral01|EQ_EllipticIntegral01]] | |||
</td> | |||
<td align="center"> | |||
<font size="+1" color="darkblue">… of the First Kind</font><br /> | |||
{{User:Tohline/Math/EQ_EllipticIntegral01}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* [https://dlmf.nist.gov/19.5.E1 DLMF §19.5.1] <br /> | |||
* [https://mathworld.wolfram.com/CompleteEllipticIntegraloftheFirstKind.html Wolfram's Mathworld]<br /> | |||
* [https://en.wikipedia.org/wiki/Elliptic_integral#Complete_elliptic_integral_of_the_first_kind Wikipedia]<br /> | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_EllipticIntegral03|EQ_EllipticIntegral03]] | |||
</td> | |||
<td align="center"> | |||
<font size="+1" color="darkblue">… of the First Kind</font><font color="darkblue"> (alternate expression)</font><br /> | |||
{{User:Tohline/Math/EQ_EllipticIntegral03}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_EllipticIntegral02|EQ_EllipticIntegral02]] | |||
</td> | |||
<td align="center"> | |||
<font size="+1" color="darkblue">… of the Second Kind</font><br /> | |||
{{User:Tohline/Math/EQ_EllipticIntegral02}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* [https://dlmf.nist.gov/19.5.E2 DLMF §19.5.2] <br /> | |||
* [https://mathworld.wolfram.com/CompleteEllipticIntegraloftheSecondKind.html Wolfram's MathWorld]<br /> | |||
* [https://en.wikipedia.org/wiki/Elliptic_integral#Complete_elliptic_integral_of_the_second_kind Wikipedia] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_EllipticIntegral04|EQ_EllipticIntegral04]] | |||
</td> | |||
<td align="center"> | |||
<font size="+1" color="darkblue">… of the Second Kind</font><font color="darkblue"> (alternate expression)</font><br /> | |||
{{User:Tohline/Math/EQ_EllipticIntegral04}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* | |||
</td> | |||
</tr> | |||
</table> | |||
</div> | |||
See also: | |||
* [https://www-jstor-org.libezp.lib.lsu.edu/stable/2004103?seq=1#metadata_info_tab_contents W. J. Cody (1965, Mathematics of Computation, Vol. 19, No. 89, pp. 105 - 112)], "<i>Chebyshev Approximations for the Complete Elliptic Integrals K and E</i>". | |||
* "[https://www.ams.org/journals/mcom/1965-19-090/S0025-5718-1965-0178563-0/S0025-5718-1965-0178563-0.pdf Chebyshev Polynomial Expansions of Complete Elliptic Integrals]," by W. J. Cody (Argonne National Laboratory) | |||
===Toroidal Function Evaluations=== | |||
====Analytic Expressions & Plots==== | |||
<div align="center"> | |||
<table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | |||
<tr> | |||
<th colspan=3 align="center"> | |||
<font size="+1" color="darkblue">Toroidal Function Evaluations</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td colspan=2> | |||
To insert a given equation into any Wiki document, type ...<br /><center> | |||
{{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | |||
</td> | |||
<td colspan=1 rowspan="2" align="center"> | |||
<br /> <br /> <br /> | |||
<font color="red">Graphical Representation</font> <br />(see: [[User:Tohline/Appendix/Mathematics/ToroidalFunctions#Caption|generic caption]]) | |||
</td> | |||
</tr> | |||
<tr> | |||
<th width="10%"> | |||
<font color="red">Template_Name</font> | |||
</th> | |||
<th width="75%"> | |||
<font color="red">Resulting Equation</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_PminusHalf01|EQ_PminusHalf01]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_PminusHalf01}} | |||
NOTE: We have [[User:Tohline/Apps/Wong1973Potential#Attempt_.231|explicitly demonstrated]] that an alternate, equivalent expression is: | |||
<table border="0" cellpadding="5" align="center"> | |||
<tr> | |||
<td align="right"> | |||
<math>~P_{-\frac{1}{2}}(\cosh\eta)</math> | |||
</td> | |||
<td align="center"> | |||
<math>~=</math> | |||
</td> | |||
<td align="left"> | |||
<math>~\frac{\sqrt{2}}{\pi} (\sinh\eta)^{-1 / 2} k K(k)</math> | |||
</td> | |||
<td align="center"> where: </td> | |||
<td align="right"> | |||
<math>~k</math> | |||
</td> | |||
<td align="center"> | |||
<math>~\equiv</math> | |||
</td> | |||
<td align="left"> | |||
<math>~[2/(\coth\eta + 1)]^{1 / 2} \, .</math> | |||
</td> | |||
</tr> | |||
</table> | |||
</td> | |||
<td colspan=1 align="left"> | |||
[[File:P0minus1Half3.png|200px|center|P0minus1Half]] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_QminusHalf01|EQ_QminusHalf01]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_QminusHalf01}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
[[File:Q0minus1Half3.png|200px|center|Q0minusHalf]] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_PplusHalf01|EQ_PplusHalf01]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_PplusHalf01}} | |||
NOTE: It appears as though an alternate, equivalent expression is: | |||
<table border="0" cellpadding="5" align="center"> | |||
<tr> | |||
<td align="right"> | |||
<math>~P_{+\frac{1}{2}}(\cosh\eta)</math> | |||
</td> | |||
<td align="center"> | |||
<math>~=</math> | |||
</td> | |||
<td align="left"> | |||
<math>~\frac{\sqrt{2}}{\pi} (\sinh\eta)^{+1 / 2} k^{-1} E(k)</math> | |||
</td> | |||
<td align="center"> where: </td> | |||
<td align="right"> | |||
<math>~k</math> | |||
</td> | |||
<td align="center"> | |||
<math>~\equiv</math> | |||
</td> | |||
<td align="left"> | |||
<math>~[2/(\coth\eta + 1)]^{1 / 2} \, .</math> | |||
</td> | |||
</tr> | |||
</table> | |||
</td> | |||
<td colspan=1 align="left"> | |||
[[File:P0plus1Half4.png|200px|center|P0plusHalf]] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_QplusHalf01|EQ_QplusHalf01]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_QplusHalf01}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
[[File:Q0plus1Half3.png|200px|center|Q0plusHalf]] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Q1minusHalf01|EQ_Q1minusHalf01]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Q1minusHalf01}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
[[File:ABSQ1minus1Half3.png|200px|center|ABSQ1minusHalf]] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Q2minusHalf01|EQ_Q2minusHalf01]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Q2minusHalf01}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
[[File:Q2minus1Half3.png|200px|center|Q2minusHalf]] | |||
</td> | |||
</tr> | |||
</table> | |||
</div> | |||
====Caption for Plots==== | |||
<div align="center" id="Caption"> | |||
<table border="1" cellpadding="8" width="95%" align="center"> | |||
<tr> | |||
<td align="left"> | |||
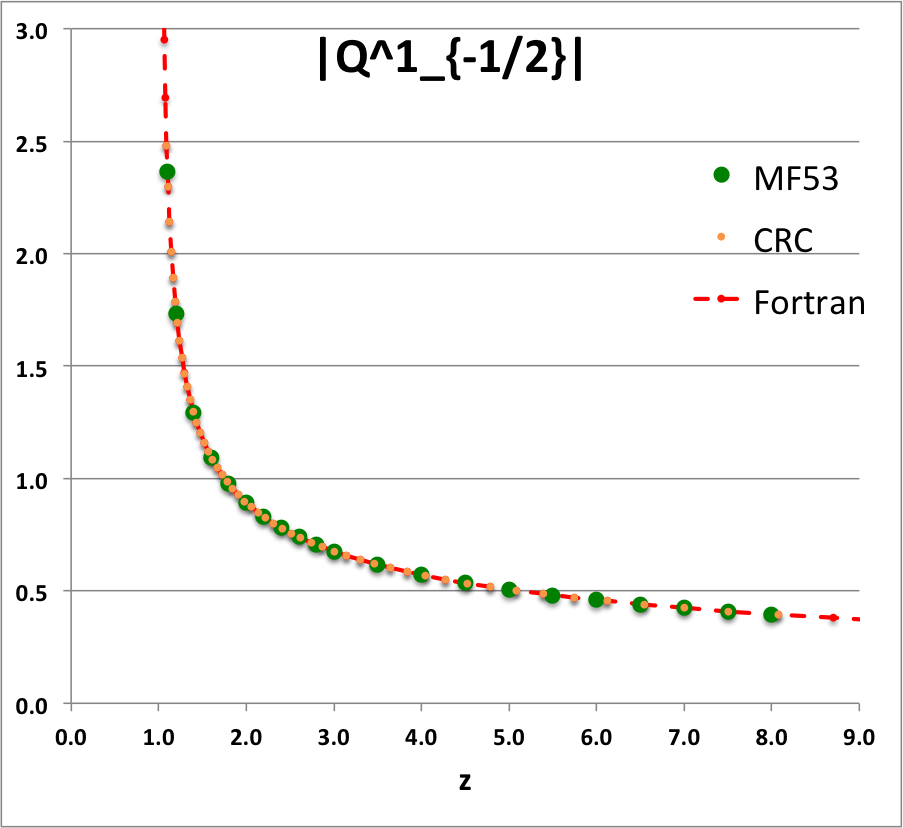
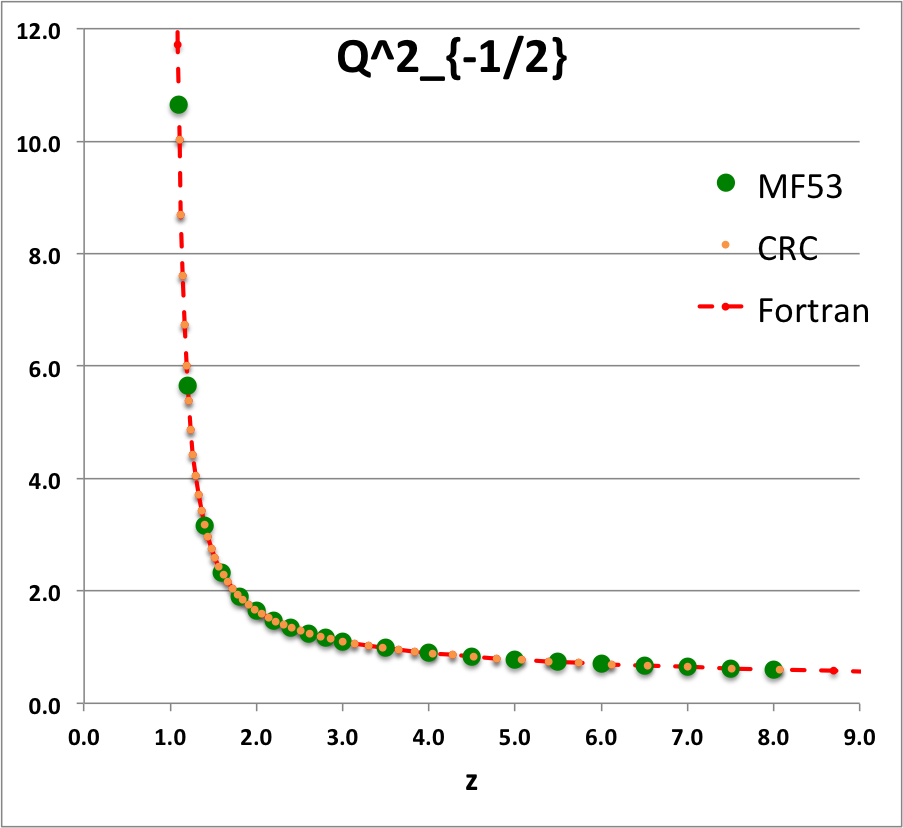
'''Caption for Plots:''' Here we explain how we assembled the various plots — shown [[#Toroidal_Function_Evaluations|immediately above]] in the right-hand column of the "Toroidal Function Evaluations" table — that depict the behavior of various associated Legendre (toroidal) functions (see the [[User:Tohline/Appendix/Mathematics/ToroidalFunctions#Summary_of_Toroidal_Coordinates_and_Toroidal_Functions|related discussion]]) having varying half-integer degrees <math>~P^0_{-\frac{1}{2}}</math>, <math>~P^0_{+\frac{1}{2}}</math>, <math>~Q^0_{-\frac{1}{2}}</math>, <math>~Q^0_{+\frac{1}{2}}</math>, <math>~Q^0_{+\frac{3}{2}} \, ,</math> and (in association with a [[User:Tohline/Appendix/Mathematics/ToroidalSynopsis01#Q1Q2Summary|separate related discussion]]) having varying order <math>~Q^1_{-\frac{1}{2}}</math>, <math>~Q^2_{-\frac{1}{2}}</math>. | |||
For each choice of the integer indexes, <math>~n \ge 0</math> and <math>~m \ge 0</math>, the relevant plot shows how the function, <math>~X^n_{m-\frac{1}{2}}(z)</math>, varies with <math>~z</math>. In each plot … | |||
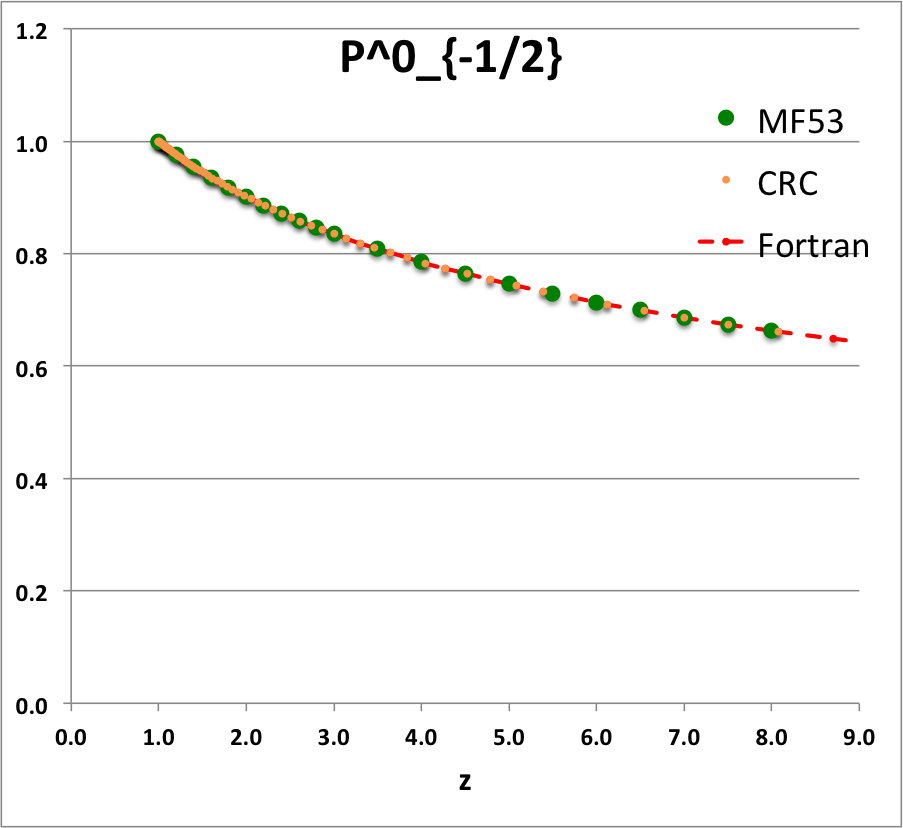
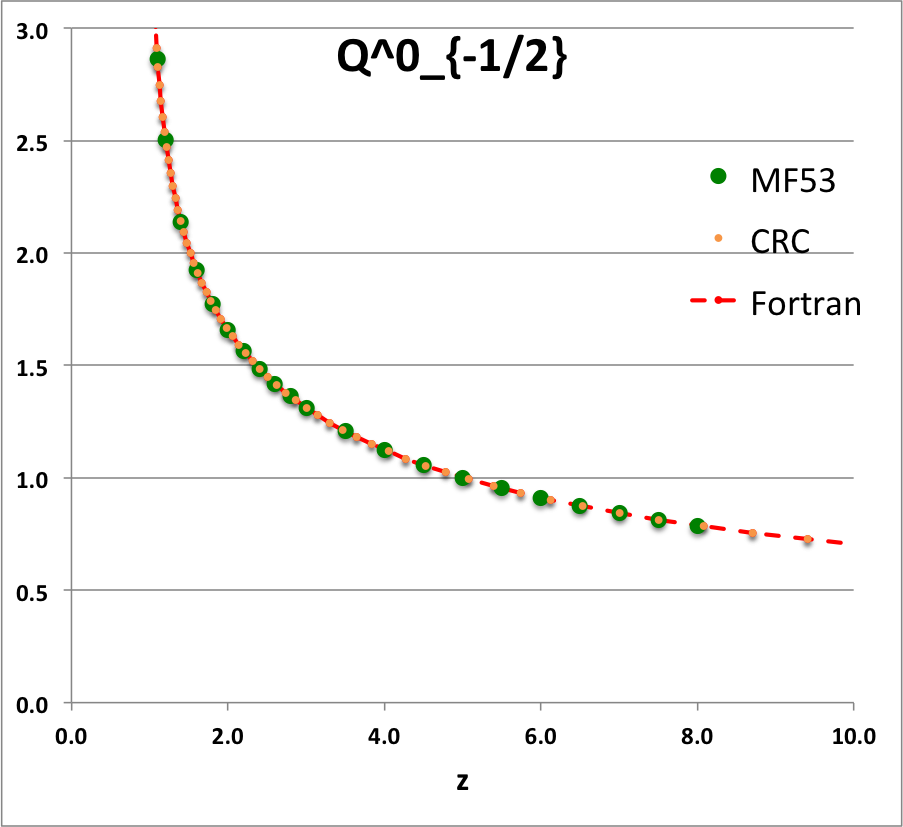
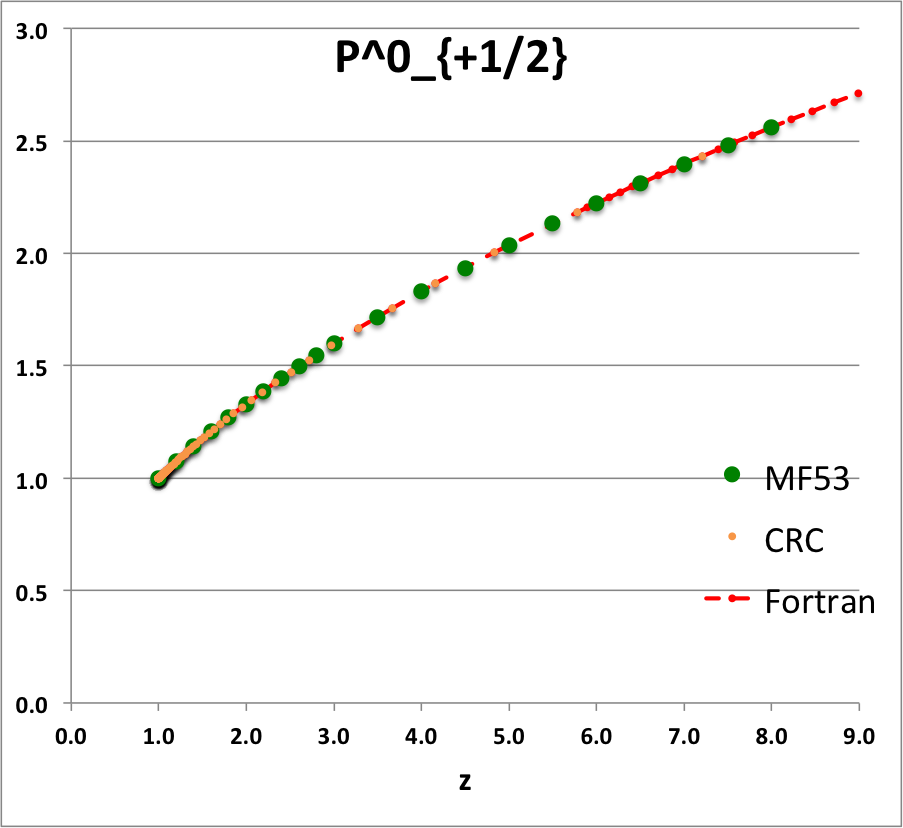
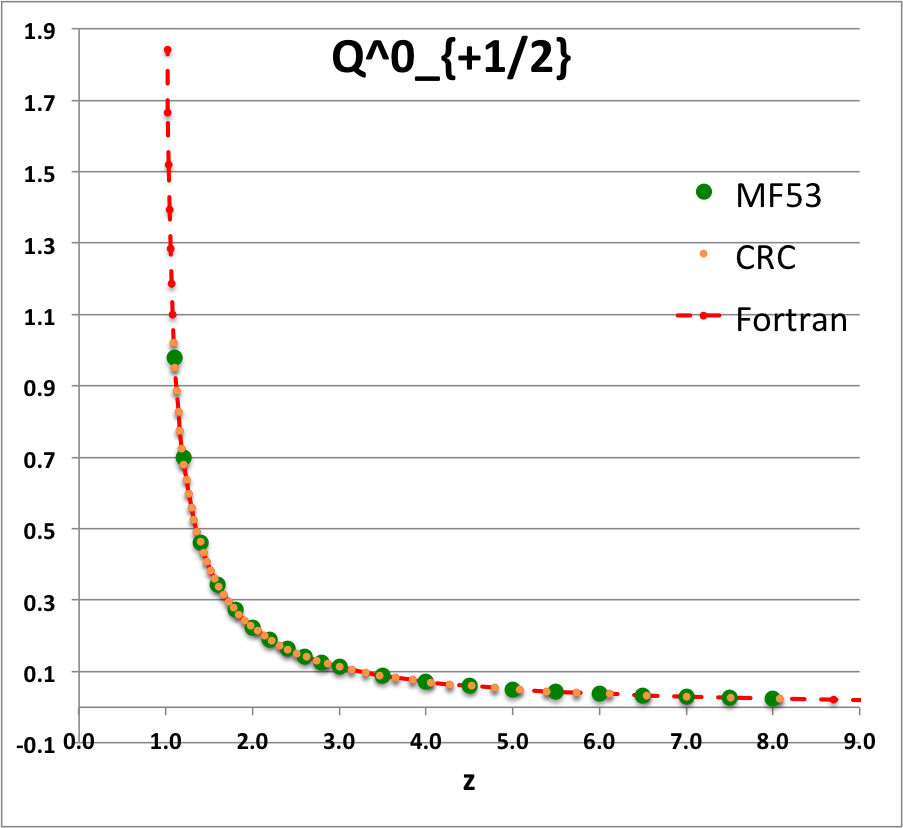
* The solid green circular markers identify data that has been pulled directly from Table IX (p. 1923) of [<b>[[User:Tohline/Appendix/References#MF53|<font color="red">MF53</font>]]</b>]; | |||
* The solid orange circular markers identify function values that we have calculated using the relevant formulae as expressed herein in terms of the complete elliptic integrals, <math>~K(k)</math> and <math>~E(k)</math>, where the relevant values of the elliptic integrals have been pulled directly from tabulated values published in pp. 535 - 537 of the 1971 (19<sup>th</sup>) edition of the CRC's ''Standard Mathematical Tables'', published by the Chemical Rubber Co., Cleveland, Ohio, U.S.A.. (See an accompanying sample of [[User:Tohline/2DStructure/ToroidalCoordinateIntegrationLimits#Evaluation_of_Elliptic_Integrals|elliptic integral values extracted from this CRC handbook]].) | |||
* The dashed red curve was also derived using formulae expressed in terms of the complete elliptic integrals, but the ''values'' of the elliptic integrals have been calculated using (double-precision versions of) algorithms drawn from ''Numerical Recipes.'' | |||
NOTE: The tabulated values of the function, <math>~Q^1_{-\frac{1}{2}}</math>, that appear in Table IX (p. 1923) of [<b>[[User:Tohline/Appendix/References#MF53|<font color="red">MF53</font>]]</b>] — see [[#Comparison_with_Table_IX_from_MF53|immediately below]] — are all positive, whereas, according to our derivation, they should all be negative. Therefore, for comparison purposes of this ''specific'' function — both here and in our [[User:Tohline/Appendix/Mathematics/ToroidalSynopsis01#Q1Q2Summary|accompanying discussion]] — we have plotted the absolute value of the function, <math>~|Q^1_{-\frac{1}{2}}(z)|</math>. | |||
ADDITIONAL NOTE: In ''Example 4'' on p. 340 of [https://books.google.com/books?id=MtU8uP7XMvoC&printsec=frontcover&dq=Abramowitz+and+stegun&hl=en&sa=X&ved=0ahUKEwialra5xNbaAhWKna0KHcLAASAQ6AEILDAA#v=onepage&q=Abramowitz%20and%20stegun&f=false Abramowitz & Stegun (1995)], we can pull one additional data point for comparison; specifically, they provide a high-precision evaluation of <math>~Q^0_{-\frac{1}{2}}(z = 2.6) = 1.419337751</math>. As can be seen in the [[#Comparison_with_Table_IX_from_MF53|table of function values immediately below]], this is entirely consistent with the lower-precision value that we have extracted from [<b>[[User:Tohline/Appendix/References#MF53|<font color="red">MF53</font>]]</b>], and exactly matches the double-precision value we have calculated based on the ''Numerical Recipes'' algorithm. | |||
</td> | |||
</tr> | |||
</table> | |||
</div> | |||
====Example Recurrence Relations==== | |||
The above [[#Analytic_Expressions_.26_Plots|''Toroidal Function Evaluations'']] table provides analytic expressions for the pair of foundation functions, <math>~P^0_{-\frac{1}{2}}(z)</math> and <math>~P^0_{+\frac{1}{2}}(z)</math>, and the associated pair of foundation functions, <math>~Q^0_{-\frac{1}{2}}(z)</math> and <math>~Q^0_{+\frac{1}{2}}(z)</math>. From either pair of foundation functions, expressions for all other zero-order, half-integer degree toroidal functions can be obtained using a relatively simple recurrence relation drawn from the "Key Equation," | |||
{{ User:Tohline/Math/EQ_Toroidal04 }} | |||
Specifically, letting <math>~\mu \rightarrow 0</math> and <math>~\nu \rightarrow (m - \tfrac{1}{2})</math>, for all <math>~m \ge 2</math>, we have, | |||
<table border="0" cellpadding="5" align="center"> | |||
<tr> | |||
<td align="right"> | |||
<math>~P^0_{m-\frac{1}{2}}(z)</math> | |||
</td> | |||
<td align="center"> | |||
<math>~=</math> | |||
</td> | |||
<td align="left"> | |||
<math>~4 \biggl[ \frac{m-1}{2m-1} \biggr] z P^0_{m-\frac{3}{2}}(z) - \biggl[ \frac{2m-3}{2m-1}\biggr]P^0_{m-\frac{5}{2}}(z) \, ;</math> and, | |||
</td> | |||
</tr> | |||
<tr> | |||
<td align="right"> | |||
<math>~Q^0_{m-\frac{1}{2}}(z)</math> | |||
</td> | |||
<td align="center"> | |||
<math>~=</math> | |||
</td> | |||
<td align="left"> | |||
<math>~4 \biggl[ \frac{m-1}{2m-1} \biggr] z Q^0_{m-\frac{3}{2}}(z) - \biggl[ \frac{2m-3}{2m-1}\biggr]Q^0_{m-\frac{5}{2}}(z) \, .</math> | |||
</td> | |||
</tr> | |||
</table> | |||
As examples, these two relations have been used to generate columns of numbers in the [[#Comparison_with_Table_IX_from_MF53|comparison table shown below]] for, respectively, the toroidal functions, <math>~P^0_{+\frac{3}{2}}(z)</math> and <math>~Q^0_{+\frac{3}{2}}(z)</math>. For order-1 and order-2 toroidal functions, the above table provides analytic expressions only for (the functions of the lowest half-integer degree) <math>~Q^1_{-\frac{1}{2}}(z)</math> and <math>~Q^2_{-\frac{1}{2}}(z)</math>. But, as we have detailed in an [[User:Tohline/Appendix/Mathematics/ToroidalSynopsis01#Evaluating_Q2.CE.BD|accompanying discussion]], additional order-1 and order-2 expressions can be straightforwardly derived by drawing upon another key recurrence relation, namely, | |||
{{ User:Tohline/Math/EQ_Toroidal07 }} | |||
Specifically, after adopting the association, <math>~\nu \rightarrow (n - \tfrac{1}{2})</math>, we have, when <math>~\mu = 0</math>, | |||
<table border="0" cellpadding="5" align="center"> | |||
<tr> | |||
<td align="right"> | |||
<math>~Q_{n - \frac{1}{2}}^{1}(z)</math> | |||
</td> | |||
<td align="center"> | |||
<math>~=</math> | |||
</td> | |||
<td align="left"> | |||
<math>~ | |||
(n-\tfrac{1}{2}) (z^2-1)^{-\frac{1}{2}} [z Q_{n - \frac{1}{2}}(z) - Q_{n - \frac{3}{2}}(z)] | |||
</math> | |||
</td> | |||
<td allign="center"> … </td> | |||
<td align="left"> | |||
for <math>~n \ge 1 \, ,</math> | |||
</td> | |||
</tr> | |||
</table> | |||
and, when <math>~\mu = 1</math>, | |||
<table border="0" cellpadding="5" align="center"> | |||
<tr> | |||
<td align="right"> | |||
<math>~Q_{n - \frac{1}{2}}^{2}(z)</math> | |||
</td> | |||
<td align="center"> | |||
<math>~=</math> | |||
</td> | |||
<td align="left"> | |||
<math>~ | |||
(z^2-1)^{-\frac{1}{2}} \{ (n-\tfrac{3}{2}) z Q^1_{n - \frac{1}{2}}(z) - (n+\tfrac{1}{2})Q^1_{n - \frac{3}{2}}(z)\} | |||
</math> | |||
</td> | |||
<td allign="center"> … </td> | |||
<td align="left"> | |||
for <math>~n \ge 1 \, .</math> | |||
</td> | |||
</tr> | |||
</table> | |||
As an example, the first of these two relations has been used to generate a column of numbers in the [[#Comparison_with_Table_IX_from_MF53|comparison table shown below]] for the toroidal function, <math>~Q^1_{+\frac{1}{2}}(z)</math>. | |||
====Comparison with Table IX from MF53==== | |||
To facilitate ''copying & pasting'' for immediate use by other researchers, here we present in a tab-delimited, plain-text format the evaluation of nine separate toroidal functions: (''Top half of table'') <math>~P^0_{-\frac{1}{2}}</math>, <math>~P^0_{+\frac{1}{2}}</math> and <math>~P^0_{+\frac{3}{2}}</math>; (''Bottom half of table'') <math>~Q^0_{-\frac{1}{2}}</math>, <math>~Q^1_{-\frac{1}{2}}</math>, <math>~Q^2_{-\frac{1}{2}}</math>, <math>~Q^0_{+\frac{1}{2}}</math>, <math>~Q^1_{+\frac{1}{2}}</math> and <math>~Q^0_{+\frac{3}{2}}</math>. Each function has been evaluated for approximately 23 different argument values in the range, <math>~1.0 \le z \le 9.0</math>, and, for each function, two columns of function values have been presented: (''Left column'') Low-precision evaluation extracted directly from Table IX (p. 1923) of [<b>[[User:Tohline/Appendix/References#MF53|<font color="red">MF53</font>]]</b>]; (''Right column'') Our double-precision evaluation based on a set of ''Numerical Recipes'' algorithms. One exception: The value listed under the "MF53" column for the evaluation of <math>~Q^0_{-\frac{1}{2}}(z=2.6)</math> is the high-precision value published on p. 340 of [https://books.google.com/books?id=MtU8uP7XMvoC&printsec=frontcover&dq=Abramowitz+and+stegun&hl=en&sa=X&ved=0ahUKEwialra5xNbaAhWKna0KHcLAASAQ6AEILDAA#v=onepage&q=Abramowitz%20and%20stegun&f=false Abramowitz & Stegun (1995)]; notice that our high-precision evaluation matches all ten digits of their published value. | |||
<div align="center" id="TabulatedValues"> | |||
<table border="1" cellpadding="8" width="50%" align="center"> | |||
<tr> | |||
<td align="center"> | |||
Top half of Table IX (p. 1923) of [<b>[[User:Tohline/Appendix/References#MF53|<font color="red">MF53</font>]]</b>] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td align="left"> | |||
<pre> | |||
z P0m1Half(z) P0p1Half(z) P0p3Half(z) | |||
MF53 Our Calc. MF53 Our Calc. MF53 Our Calc. | |||
1.0 1.0000 1.0000 1.0000 | |||
1.2 0.9763 9.763155118E-01 1.0728 1.072784040E+00 1.3910 1.391015961E+00 | |||
1.4 0.9549 9.549467781E-01 1.1416 1.141585331E+00 1.8126 1.812643692E+00 | |||
1.6 0.9355 9.355074856E-01 1.2070 1.206963827E+00 2.2630 2.263020336E+00 | |||
1.8 0.9177 9.176991005E-01 1.2694 1.269362428E+00 2.7406 2.740570128E+00 | |||
2.0 0.9013 9.012862994E-01 1.3291 1.329138155E+00 3.2439 3.243939648E+00 | |||
2.2 0.8861 8.860804115E-01 1.3866 1.386583505E+00 3.7719 3.771951476E+00 | |||
2.4 0.8719 8.719279330E-01 1.4419 1.441941436E+00 4.3236 4.323569952E+00 | |||
2.6 0.8587 8.587023595E-01 1.4954 1.495416274E+00 4.8979 4.897875630E+00 | |||
2.8 0.8463 8.462982520E-01 1.5472 1.547181667E+00 5.4941 5.494045473E+00 | |||
3.0 0.8346 8.346268417E-01 1.5974 1.597386605E+00 6.1113 6.111337473E+00 | |||
3.5 0.8082 8.081851582E-01 1.7169 1.716877977E+00 7.7427 7.742702172E+00 | |||
4.0 0.7850 7.849616703E-01 1.8290 1.828992729E+00 9.4930 9.492973996E+00 | |||
4.5 0.7643 7.643076802E-01 1.9349 1.934919997E+00 11.3555 1.135475076E+01 | |||
5.0 0.7457 7.457491873E-01 2.0356 2.035563839E+00 13.3220 1.332184253E+01 | |||
5.5 0.7289 7.289297782E-01 2.1316 2.131629923E+00 15.3890 1.538897617E+01 | |||
6.0 0.7136 7.135750093E-01 2.2237 2.223681177E+00 17.5520 1.755159108E+01 | |||
6.5 0.6995 6.994692725E-01 2.3122 2.312174942E+00 19.8060 1.980569307E+01 | |||
7.0 0.6864 6.864402503E-01 2.3975 2.397488600E+00 22.1480 2.214774685E+01 | |||
7.5 0.6743 6.743481630E-01 2.4799 2.479937758E+00 24.5750 2.457459486E+01 | |||
8.0 0.6631 6.630781433E-01 2.5598 2.559789460E+00 27.0830 2.708339486E+01 | |||
8.5 6.525347093E-01 2.637271986E+00 2.967157094E+01 | |||
9.0 6.426376817E-01 2.712582261E+00 3.233677457E+01 | |||
</pre> | |||
</td> | |||
</tr> | |||
<tr> | |||
<td align="center"> | |||
Bottom half of Table IX (p. 1923) of [<b>[[User:Tohline/Appendix/References#MF53|<font color="red">MF53</font>]]</b>] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td align="left"> | |||
<pre> | |||
z Q0m1Half(z) Q1m1Half(z) Q2m1Half(z) Q0p1Half(z) Q1p1Half(z) Q0p3Half(z) | |||
MF53 Our Cal. MF53 Our Calc. MF53 Our Calc. M53 Our Calc. MF53 Our Calc. MF53 Our Calc. | |||
1.1 2.8612 2.861192872E+00 2.3661 -2.366084077E+00 10.6440 1.064378304E+01 0.9788 9.787602829E-01 1.9471 -1.947110839E+00 0.4818 4.817841242E-01 | |||
1.2 2.5010 2.500956508E+00 1.7349 -1.734890983E+00 5.6518 5.651832631E+00 0.6996 6.995548314E-01 1.2524 -1.252395745E+00 0.2856 2.856355610E-01 | |||
1.4 2.1366 2.136571733E+00 1.2918 -1.291802851E+00 3.1575 3.157491205E+00 0.4598 4.597941602E-01 0.7618 -7.618218821E-01 0.14609 1.460918547E-01 | |||
1.6 1.9229 1.922920866E+00 1.0943 -1.094337965E+00 2.3230 2.323018870E+00 0.3430 3.430180260E-01 0.5501 -5.500770475E-01 0.09080 9.079816684E-02 | |||
1.8 1.7723 1.772268479E+00 0.9748 -9.748497733E-01 1.9018 1.901788930E+00 0.2720 2.720401772E-01 0.4285 -4.284853031E-01 0.06214 6.214026586E-02 | |||
2.0 1.6566 1.656638170E+00 0.8918 -8.917931374E-01 1.6454 1.645348489E+00 0.2240 2.240142929E-01 0.3489 -3.488955345E-01 0.04516 4.515872426E-02 | |||
2.2 1.5634 1.563378886E+00 0.8293 -8.292825549E-01 1.4712 1.471197798E+00 0.18932 1.893229696E-01 0.29263 -2.926294028E-01 0.03422 3.422108228E-02 | |||
2.4 1.4856 1.485653983E+00 0.7798 -7.797558474E-01 1.3441 1.344108936E+00 0.16312 1.631167365E-01 0.25076 -2.507568731E-01 0.02676 2.675556229E-02 | |||
2.6 1.419337751 1.419337751E+00 0.7391 -7.390875295E-01 1.2465 1.246521876E+00 0.14266 1.426580119E-01 0.21842 -2.184222751E-01 0.02143 2.143519083E-02 | |||
2.8 1.3617 1.361744950E+00 0.7048 -7.048053314E-01 1.1687 1.168702464E+00 0.12628 1.262756033E-01 0.19274 -1.927423405E-01 0.01751 1.751393553E-02 | |||
3.0 1.3110 1.311028777E+00 0.6753 -6.753219405E-01 1.1048 1.104816977E+00 0.11289 1.128885424E-01 0.17189 -1.718911443E-01 0.01454 1.454457729E-02 | |||
3.5 1.2064 1.206444997E+00 0.6163 -6.163068170E-01 0.9846 9.846190928E-01 0.08824 8.824567577E-02 0.13380 -1.338040913E-01 0.00966 9.664821286E-03 | |||
4.0 1.1242 1.124201960E+00 0.5713 -5.712994484E-01 0.8990 8.990205764E-01 0.07154 7.154134054E-02 0.10819 -1.081900595E-01 0.00682 6.819829619E-03 | |||
4.5 1.0572 1.057164923E+00 0.5353 -5.353494651E-01 0.8339 8.338659751E-01 0.05957 5.956966068E-02 0.08993 -8.992645608E-02 0.00503 5.029656514E-03 | |||
5.0 1.0011 1.001077380E+00 0.5057 -5.056928088E-01 0.7820 7.819717783E-01 0.05063 5.062950976E-02 0.07634 -7.633526879E-02 0.00384 3.837604899E-03 | |||
5.5 0.9532 9.532056775E-01 0.4806 -4.806378723E-01 0.7393 7.392682950E-01 0.04374 4.373774515E-02 0.06588 -6.588433822E-02 0.00301 3.008238619E-03 | |||
6.0 0.9117 9.116962715E-01 0.4591 -4.590784065E-01 0.7033 7.032568965E-01 0.03829 3.828867029E-02 0.05764 -5.763649873E-02 0.00241 2.410605139E-03 | |||
6.5 0.87524 8.752387206E-01 0.44025 -4.402537373E-01 0.67231 6.723067009E-01 0.03389 3.389003482E-02 0.05099 -5.098806037E-02 0.00197 1.967394932E-03 | |||
7.0 0.84288 8.428751774E-01 0.42362 -4.236198508E-01 0.64530 6.453008278E-01 0.03028 3.027740449E-02 0.04553 -4.553369214E-02 0.00163 1.630716095E-03 | |||
7.5 0.81389 8.138862008E-01 0.40877 -4.087751846E-01 0.62144 6.214442864E-01 0.02727 2.726650960E-02 0.04099 -4.099183107E-02 0.00137 1.369695722E-03 | |||
8.0 0.78772 7.877190099E-01 0.39542 -3.954155185E-01 0.60015 6.001530105E-01 0.02473 2.472532098E-02 0.03716 -3.716124286E-02 0.00116 1.163753807E-03 | |||
8.5 7.639406230E-01 -3.833053056E-01 5.809864341E-01 2.255696890E-02 -3.389458114E-02 9.987731857E-04 | |||
9.0 7.422062367E-01 -3.722587645E-01 5.636047532E-01 2.068890884E-02 -3.108168349E-02 8.648271474E-04 | |||
</pre> | |||
</td> | |||
</tr> | |||
</table> | |||
</div> | |||
===Relationships Between Various Associated Legendre Functions=== | |||
<div align="center"> | |||
<table border=3 cellpadding=5 cellspacing=1 width="95%" bordercolor="darkblue"> | |||
<tr> | |||
<th colspan=3 align="center"> | |||
<font size="+1" color="darkblue">Relationships Between Various Associated Legendre Functions</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td colspan=2> | |||
To insert a given equation into any Wiki document, type ...<br /><center> | |||
{{ User:Tohline/Math/<i><font color="red">Template_Name</font></i> }}</center> | |||
</td> | |||
<td colspan=1 rowspan="2" align="center"> | |||
<br /> <br /> <br /> | |||
<font color="red">See also …</font> | |||
</td> | |||
</tr> | |||
<tr> | |||
<th width="10%"> | |||
<font color="red">Template_Name</font> | |||
</th> | |||
<th width="75%"> | |||
<font color="red">Resulting Equation</font> | |||
</th> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Toroidal01|EQ_Toroidal01]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Toroidal01}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* [http://adsabs.harvard.edu/abs/1940QJMat..11..222C T. G. Cowling (1940)]: p. 223 (note sign discrepancy in argument of <math>~Q_\nu</math>) | |||
* [https://dlmf.nist.gov/14.18.E5 DLMF §14.18.5] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Toroidal02|EQ_Toroidal02]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Toroidal02}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* [http://adsabs.harvard.edu/abs/2000AN....321..363C Cohl et al. (2000)], eq. (34) | |||
* [https://dlmf.nist.gov/14.19#v DLMF §14.19.v] together with [https://dlmf.nist.gov/14.3.E10 DLMF §14.3.10] | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Toroidal03|EQ_Toroidal03]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Toroidal03}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
| |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Toroidal04|EQ_Toroidal04]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Toroidal04}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* [http://adsabs.harvard.edu/abs/1999ApJ...527...86C Cohl & Tohline (1999)], §2.2.2, eq. (25)<br /> | |||
* [https://dl-acm-org.libezp.lib.lsu.edu/citation.cfm?id=365474&picked=prox Guatschi (1965)], p. 490, '''procedure''' ''toroidal | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Toroidal05|EQ_Toroidal05]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Toroidal05}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Toroidal06|EQ_Toroidal06]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Toroidal06}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* [https://authors.library.caltech.edu/43491/1/Volume%201.pdf Erdélyi (1953)]: Volume I, §3.8, p. 162, eq. (21) | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Toroidal07|EQ_Toroidal07]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Toroidal07}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Toroidal08|EQ_Toroidal08]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Toroidal08}} | |||
</td> | |||
<td colspan=1 align="left"> | |||
* | |||
</td> | |||
</tr> | |||
</table> | |||
</div> | |||
<br /> | |||
<span id="KPR"> | |||
=<font size="+2" color="darkblue">Key Parallel References (printed texts spanning 5+ decades)</font>= | |||
</span> | |||
* [<b><font color="red">C67</font></b>] [http://adsabs.harvard.edu/abs/1967aits.book.....C '''Chandrasekhar, S.''' 1967] (originally, 1939), An Introduction to the Study of Stellar Structure (New York: Dover) | |||
** <span id="C67note_EOSideal0A">EQ_EOSideal0A</span> — In C67, the ideal gas equation of state is initially written in terms of the specific volume {{User:Tohline/Math/VAR_SpecificVolume01}}, instead of the mass density {{User:Tohline/Math/VAR_Density01}}; also, it is initially assumed that {{User:Tohline/Math/MP_MeanMolecularWeight}} = 1. Both {{User:Tohline/Math/VAR_Density01}} and {{User:Tohline/Math/MP_MeanMolecularWeight}} are introduced in §III.1, Eq.(5). | ** <span id="C67note_EOSideal0A">EQ_EOSideal0A</span> — In C67, the ideal gas equation of state is initially written in terms of the specific volume {{User:Tohline/Math/VAR_SpecificVolume01}}, instead of the mass density {{User:Tohline/Math/VAR_Density01}}; also, it is initially assumed that {{User:Tohline/Math/MP_MeanMolecularWeight}} = 1. Both {{User:Tohline/Math/VAR_Density01}} and {{User:Tohline/Math/MP_MeanMolecularWeight}} are introduced in §III.1, Eq.(5). | ||
** <span id="C67note_SSLaneEmden01">EQ_SSLaneEmden01</span> — At the end of his Chapter IV, C67 writes an extensive history of the earliest work on stellar structure pointing especially the origins of the so-called Lane-Emden equation. He points out, for example, that [http://gallica.bnf.fr/ark:/12148/bpt6k152556/f340.image.r=Annalen%20der%20Physic.langEN Ritter (1880)] actually published this governing differential equation prior to Emden. | |||
* [<b><font color="red">LL75</font></b>] '''Laundau, L. D. & Lifshitz, E. M.''' 1975 (originally, 1959), Fluid Mechanics (New York: Pergamon Press) | * [<b><font color="red">LL75</font></b>] [http://adsabs.harvard.edu/abs/1959flme.book.....L '''Laundau, L. D. & Lifshitz, E. M.''' 1975 (originally, 1959)], Fluid Mechanics (New York: Pergamon Press) | ||
** <span id="LL75note_Continuity01">EQ_Continuity01</span> — LL75 present the Eulerian, rather than the Lagrangian form of the Continuity equation. | ** <span id="LL75note_Continuity01">EQ_Continuity01</span> — LL75 present the Eulerian, rather than the Lagrangian form of the Continuity equation. | ||
** <span id="LL75note_Euler01">EQ_Euler01</span> — In the Euler equation, LL75 do not initially include a source term to account for a gradient in the Newtonian gravitational potential, {{User:Tohline/Math/VAR_NewtonianPotential01}}; a term representing acceleration due to gravity, <math>\vec{g} = -\nabla\Phi</math>, is introduced in Eq.(2.4), but in LL75 this is intended primarily to describe gravity at the surface of the Earth. | ** <span id="LL75note_Euler01">EQ_Euler01</span> — In the Euler equation, LL75 do not initially include a source term to account for a gradient in the Newtonian gravitational potential, {{User:Tohline/Math/VAR_NewtonianPotential01}}; a term representing acceleration due to gravity, <math>\vec{g} = -\nabla\Phi</math>, is introduced in Eq.(2.4), but in LL75 this is intended primarily to describe gravity at the surface of the Earth. | ||
| Line 325: | Line 1,395: | ||
* [<b><font color="red">H87</font></b>] '''Huang, K.''' 1987 (originally 1963), Statistical Mechanics (New York: John Wiley & Sons) | * [<b><font color="red">ST83</font></b>] [http://adsabs.harvard.edu/abs/1983bhwd.book.....S '''Shapiro, S. L. & Teukolsky, S. A.''' 1983], Black Holes, White Dwarfs, and Neutron Stars: The Physics of Compact Objects (New York: John Wiley & Sons); republished in 2004 by WILEY-VCH Verlag GmbH & Co. KGaA | ||
** <span id="ST83note_Euler01">EQ_Continuity01</span> — ST83 present the Eulerian, rather than the Lagrangian form of the Continuity equation. | |||
* [<b><font color="red">H87</font></b>] [http://adsabs.harvard.edu/abs/1987stme.book.....H '''Huang, K.''' 1987] (originally 1963), Statistical Mechanics (New York: John Wiley & Sons) | |||
** <span id="H87note_Euler01">EQ_Continuity01</span> — H87 presents the Eulerian, rather than the Lagrangian form of the Continuity equation, and the variable <math>\vec{u}</math> is used instead of {{User:Tohline/Math/VAR_VelocityVector01}} to represent the velocity. | ** <span id="H87note_Euler01">EQ_Continuity01</span> — H87 presents the Eulerian, rather than the Lagrangian form of the Continuity equation, and the variable <math>\vec{u}</math> is used instead of {{User:Tohline/Math/VAR_VelocityVector01}} to represent the velocity. | ||
** <span id="H87note_Continuity01">EQ_Euler01</span> — H87 presents the Eulerian, rather than the Lagrangian form of the Euler equation, and the variable <math>\vec{u}</math> is used instead of {{User:Tohline/Math/VAR_VelocityVector01}} to represent the velocity. Furthermore, to match the source term in our version of the Euler equation, we must set H87's applied acceleration, <math>\vec{F}/m = -\nabla</math>{{User:Tohline/Math/VAR_NewtonianPotential01}}. | ** <span id="H87note_Continuity01">EQ_Euler01</span> — H87 presents the Eulerian, rather than the Lagrangian form of the Euler equation, and the variable <math>\vec{u}</math> is used instead of {{User:Tohline/Math/VAR_VelocityVector01}} to represent the velocity. Furthermore, to match the source term in our version of the Euler equation, we must set H87's applied acceleration, <math>\vec{F}/m = -\nabla</math>{{User:Tohline/Math/VAR_NewtonianPotential01}}. | ||
| Line 338: | Line 1,412: | ||
** <span id="KW94note_FirstLaw01">EQ_FirstLaw01</span> — In KW94, the symbol <math>u</math> instead of {{User:Tohline/Math/VAR_SpecificInternalEnergy01}} is used to represent the specific internal energy. | ** <span id="KW94note_FirstLaw01">EQ_FirstLaw01</span> — In KW94, the symbol <math>u</math> instead of {{User:Tohline/Math/VAR_SpecificInternalEnergy01}} is used to represent the specific internal energy. | ||
** <span id="KW94note_EOSideal0A">EQ_EOSideal0A</span> — In KW94, the ideal gas equation of state is actually first introduced in §2.2, Eq.(27), but it is seriously discussed in Chapter 13. KW94 provide a particularly nice explanation of how to calculate the model parameter, {{User:Tohline/Math/MP_MeanMolecularWeight}}. | ** <span id="KW94note_EOSideal0A">EQ_EOSideal0A</span> — In KW94, the ideal gas equation of state is actually first introduced in §2.2, Eq.(27), but it is seriously discussed in Chapter 13. KW94 provide a particularly nice explanation of how to calculate the model parameter, {{User:Tohline/Math/MP_MeanMolecularWeight}}. | ||
** <span id="KW94note_SShydrostaticBalance">EQ_SShydrostaticBalance01</span> — In KW94, the hydrostatic balance equation is expressed in terms of <math>dP/dM_r</math> instead of <math>dP/dr</math>; and the second term on the right-hand-side allows for a net radial acceleration. | |||
* [<b><font color="red">HK94</font></b>] '''Hansen, C. J. & Kawaler, S. D.''' 1994, Stellar Interiors: Physical Principles, Structure, and Evolution (New York: Springer) | |||
| Line 343: | Line 1,421: | ||
** <span id="P00note_Poisson01">EQ_Poisson01</span> — See also Vol.I: §10.4, Eq.(10.58). | ** <span id="P00note_Poisson01">EQ_Poisson01</span> — See also Vol.I: §10.4, Eq.(10.58). | ||
* [<b><font color="red">BLRY07</font></b>] <span id="BLRY07">'''Bodenheimer, P., Laughlin, G. P., Różyczka, M. & Yorke, H. W.''' 2007,</span> Numerical Methods in Astrophysics <font size="-1">An Introduction</font> (New York: Taylor & Francis) | |||
=<font size="+2" color="darkblue">Other Equations with Assigned Templates</font>= | |||
<div align="center"> | <div align="center"> | ||
| Line 356: | Line 1,436: | ||
<tr> | <tr> | ||
<th width=" | <th width="15%"> | ||
<font color="red">Template_Name</font> | <font color="red">Template_Name</font> | ||
</th> | </th> | ||
| Line 369: | Line 1,449: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_Continuity02|EQ_Continuity02]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 381: | Line 1,461: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_Euler02|EQ_Euler02]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 394: | Line 1,474: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_Euler03|EQ_Euler03]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 401: | Line 1,481: | ||
<td colspan=6> | <td colspan=6> | ||
Conservative form of the Euler equation. | Conservative form of the Euler equation. | ||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Euler04|EQ_Euler04]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Euler04}} | |||
</td> | |||
<td colspan=6> | |||
Euler equation in terms of vorticity. | |||
</td> | </td> | ||
</tr> | </tr> | ||
| Line 408: | Line 1,501: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_FirstLaw02|EQ_FirstLaw02]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 421: | Line 1,514: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_Polytrope01|EQ_Polytrope01]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 433: | Line 1,526: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_Polytrope02|EQ_Polytrope02]] | ||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Polytrope02}} | |||
</td> | |||
<td colspan=6> | |||
Enthalpy in a polytrope. | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_Polytrope03|EQ_Polytrope03]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_Polytrope03}} | |||
</td> | |||
<td colspan=6> | |||
Density in terms of enthalpy for polytrope. | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_EOSideal00|EQ_EOSideal00]] | |||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 446: | Line 1,563: | ||
<tr> | <tr> | ||
<td> | <td> | ||
[ | [[User:Tohline/Math/EQ_EOSideal02|EQ_EOSideal02]] | ||
</td> | </td> | ||
<td align="center"> | <td align="center"> | ||
| Line 453: | Line 1,570: | ||
<td colspan=6> | <td colspan=6> | ||
Alternate form of the ideal gas equation of state. | Alternate form of the ideal gas equation of state. | ||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_TRApproximation|EQ_TRApproximation]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_TRApproximation}} | |||
</td> | |||
<td colspan=6> | |||
Gravitational potential exterior to an axisymmetric torus,<br />in the [[User:Tohline/Apps/DysonWongTori#TRApproximation|Thin Ring (TR) Approximation]]. | |||
</td> | |||
</tr> | |||
<tr> | |||
<td> | |||
[[User:Tohline/Math/EQ_CT99Axisymmetric|EQ_CT99Axisymmetric]] | |||
</td> | |||
<td align="center"> | |||
{{User:Tohline/Math/EQ_CT99Axisymmetric}} | |||
</td> | |||
<td colspan=6> | |||
Gravitational potential of any axisymmetric mass distribution. | |||
</td> | </td> | ||
</tr> | </tr> | ||
Latest revision as of 17:40, 19 July 2020
 Key Equations
Key Equations

|
|---|
| | Tiled Menu | Tables of Content | Banner Video | Tohline Home Page | |
Each of the equations displayed in the Tables, below, encapsulates a physical concept that is fundamental to our understanding of — and, hence our discussion of — the structure, stability, and dynamics of self-gravitating fluids. The pervasiveness of these physical concepts throughout astrophysics is reflected in the fact that the same equations — perhaps written in slightly different forms — appear in numerous published books and research papers. When attempting to understand the physical concept that is associated with any one of these mathematical relations, it can be helpful to read how and in what context different authors have introduced the expression in their own work. These Tables offer guides to some parallel discussions that have appeared in published texts over the past 5+ decades in connection with selected sets of key physical relations.
EXAMPLE: Suppose you want to gain a better understanding of the origin of the ideal gas equation of state, the definition of the gas constant <math>~\Re</math>, or how to determine the value of the mean molecular weight <math>~\bar{\mu}</math> of a gas. According to the Table entitled Equations of State, you will find a discussion of the ideal gas equation of state: near Eq. (1) in §II.1 of Chandrasekhar (1967); near Eq. (80.8) in §IX.80 of Landau & Lifshitz (1975); near Eq. (5.91) in Vol. I, §5.6 of Padmanabhan (2000); etc. A "note" (linked to a comment further down on this page) appears along with a table entry if the relevant equation in the cited reference contains notations or symbol names that differ significantly from the equation as displayed here.
Principal Governing Equations
|
Principal Governing Equations |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
To insert a given equation into any Wiki document, type ... |
Parallel References |
|||||||||
|
Template_Name |
Resulting Equation |
C67 |
LL75 |
H87 |
ST83 |
KW94 |
P00 |
BLRY07 |
||
|
Continuity Equation:
|
|
§I.1 |
§5.4 |
§6.1 |
§2.5 |
I: §8.5 |
§1.4 |
|||
|
Euler Equation:
|
|
§I.2 |
§5.4 |
§6.1 |
§2.5 |
I: §8.5 |
§1.4 |
|||
|
1st Law of Thermodynamics:
|
|
§I.2 |
§4.2 |
§6.1 |
§4.1 |
I: §8.5 |
|
|||
|
Poisson Equation:
|
|
§I.3 |
|
§6.1 |
§1.3 |
I: §10.2 |
Chap. 7 |
|||
Equations of State
|
Equations of State |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
To insert a given equation into any Wiki document, type ... |
Parallel References |
|||||||||||||
|
Template_Name |
Resulting Equation |
C67 |
LL75 |
H87 |
ST83 |
KW94 |
P00 |
BLRY07 |
||||||
|
Ideal Gas Equation of State:
|
§II.1 |
§IX.80 |
§1.1 |
§2.3 |
§13.0 |
I: §5.6 |
§5.4 |
|||||||
|
Degenerate Electron Pressure:
——— NOTE: ——— <math> F(\chi) = 2\chi^4 - 2\chi^2 + \cdots ~~~~~~~(\mathrm{for}~~ \chi\gg 1) </math> |
§X.1 |
|
§11.2 |
§2.3 |
§15.0 |
I: §5.9.2 |
§5.6.1 |
|||||||
|
Radiation Pressure:
|
|
|
§12.1 |
|
|
|
§5.6.1 |
|||||||
|
Normalized Total Pressure:
|
|
|
|
|
|
|
|
|||||||
Traditional Equations of (Spherical) Stellar Structure
|
Traditional Equations of (Spherical) Stellar Structure |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
To insert a given equation into any Wiki document, type ... |
Parallel References |
|||||||||
|
Template_Name |
Resulting Equation |
C67 |
LL75 |
H87 |
ST83 |
KW94 |
P00 |
BLRY07 |
||
|
Mass Conservation:
|
§IV.2 |
|
|
§3.2 |
§2.1 |
II: §2.2 |
§5.1 |
|||
|
Hydrostatic Balance:
|
§IV.2 |
|
|
§3.2 |
§1.1 |
II: §2.2 |
§5.1 |
|||
|
Polytropic Lane-Emden Equation:
|
§IV.2 |
|
|
§3.3 |
§19.2 |
I: §10.3 |
|
|||
|
Isothermal Lane-Emden Equation:
|
§IV.22 |
|
|
|
§19.8 |
I: §10.3.3 |
|
|||
|
Radiation Transport:
|
§IV.22 |
|
|
|
§5.1.2 |
II: §2.2 |
|
|||
|
Energy Conservation:
|
§IV.22 |
|
|
|
§4.2 |
II: §2.2 |
|
|||
Stability: Radial Pulsation
|
Stability: Radial Pulsation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
To insert a given equation into any Wiki document, type ... |
Parallel References |
|||||||||
|
Template_Name |
Resulting Equation |
C67 |
ST83 |
KW94 |
HK94 |
P00 |
||||
|
LAWE: Linear Adiabatic Wave (or Radial Pulsation) Equation
|
|
§6.5 |
§38.1 |
§10.1.1 |
II: §3.7.1 |
|||||
|
Polytropic LAWE:
|
|
|
|
|
|
|||||
|
Isothermal LAWE:
|
|
|
|
|
|
|||||
Special Function Relationships
Gamma Function
|
Gamma Function |
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
To insert a given equation into any Wiki document, type ... |
|
||||||||||||||||||||||||||
|
Template_Name |
Resulting Equation |
||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||
Complete Elliptic Integrals
|
Complete Elliptic Integral … |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
To insert a given equation into any Wiki document, type ... |
|
|||||||||||||
|
Template_Name |
Resulting Equation |
|||||||||||||
|
… of the First Kind
|
||||||||||||||
|
… of the First Kind (alternate expression)
|
|
|||||||||||||
|
… of the Second Kind
|
||||||||||||||
|
… of the Second Kind (alternate expression)
|
|
|||||||||||||
See also:
- W. J. Cody (1965, Mathematics of Computation, Vol. 19, No. 89, pp. 105 - 112), "Chebyshev Approximations for the Complete Elliptic Integrals K and E".
- "Chebyshev Polynomial Expansions of Complete Elliptic Integrals," by W. J. Cody (Argonne National Laboratory)
Toroidal Function Evaluations
Analytic Expressions & Plots
|
Toroidal Function Evaluations |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
To insert a given equation into any Wiki document, type ... |
|
|||||||||||||||||||||||
|
Template_Name |
Resulting Equation |
|||||||||||||||||||||||
NOTE: We have explicitly demonstrated that an alternate, equivalent expression is:
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
NOTE: It appears as though an alternate, equivalent expression is:
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Caption for Plots
|
Caption for Plots: Here we explain how we assembled the various plots — shown immediately above in the right-hand column of the "Toroidal Function Evaluations" table — that depict the behavior of various associated Legendre (toroidal) functions (see the related discussion) having varying half-integer degrees <math>~P^0_{-\frac{1}{2}}</math>, <math>~P^0_{+\frac{1}{2}}</math>, <math>~Q^0_{-\frac{1}{2}}</math>, <math>~Q^0_{+\frac{1}{2}}</math>, <math>~Q^0_{+\frac{3}{2}} \, ,</math> and (in association with a separate related discussion) having varying order <math>~Q^1_{-\frac{1}{2}}</math>, <math>~Q^2_{-\frac{1}{2}}</math>.
|
Example Recurrence Relations
The above Toroidal Function Evaluations table provides analytic expressions for the pair of foundation functions, <math>~P^0_{-\frac{1}{2}}(z)</math> and <math>~P^0_{+\frac{1}{2}}(z)</math>, and the associated pair of foundation functions, <math>~Q^0_{-\frac{1}{2}}(z)</math> and <math>~Q^0_{+\frac{1}{2}}(z)</math>. From either pair of foundation functions, expressions for all other zero-order, half-integer degree toroidal functions can be obtained using a relatively simple recurrence relation drawn from the "Key Equation,"
|
<math>~(\nu - \mu + 1)P^\mu_{\nu + 1} (z)</math> |
<math>~=</math> |
<math>~ (2\nu + 1)z P_\nu^\mu(z) - (\nu + \mu)P^\mu_{\nu-1}(z) </math> |
|
|
Abramowitz & Stegun (1995), p. 334, eq. (8.5.3) |
|||
|
NOTE: <math>~Q_\nu^\mu</math>, as well as <math>~P_\nu^\mu</math>, satisfies this same recurrence relation. |
Specifically, letting <math>~\mu \rightarrow 0</math> and <math>~\nu \rightarrow (m - \tfrac{1}{2})</math>, for all <math>~m \ge 2</math>, we have,
|
<math>~P^0_{m-\frac{1}{2}}(z)</math> |
<math>~=</math> |
<math>~4 \biggl[ \frac{m-1}{2m-1} \biggr] z P^0_{m-\frac{3}{2}}(z) - \biggl[ \frac{2m-3}{2m-1}\biggr]P^0_{m-\frac{5}{2}}(z) \, ;</math> and, |
|
<math>~Q^0_{m-\frac{1}{2}}(z)</math> |
<math>~=</math> |
<math>~4 \biggl[ \frac{m-1}{2m-1} \biggr] z Q^0_{m-\frac{3}{2}}(z) - \biggl[ \frac{2m-3}{2m-1}\biggr]Q^0_{m-\frac{5}{2}}(z) \, .</math> |
As examples, these two relations have been used to generate columns of numbers in the comparison table shown below for, respectively, the toroidal functions, <math>~P^0_{+\frac{3}{2}}(z)</math> and <math>~Q^0_{+\frac{3}{2}}(z)</math>. For order-1 and order-2 toroidal functions, the above table provides analytic expressions only for (the functions of the lowest half-integer degree) <math>~Q^1_{-\frac{1}{2}}(z)</math> and <math>~Q^2_{-\frac{1}{2}}(z)</math>. But, as we have detailed in an accompanying discussion, additional order-1 and order-2 expressions can be straightforwardly derived by drawing upon another key recurrence relation, namely,
|
<math>~P_\nu^{\mu + 1}(z)</math> |
<math>~=</math> |
<math>~ (z^2-1)^{-\frac{1}{2}} \{ (\nu - \mu) z P^\mu_\nu(z) - (\nu + \mu)P^\mu_{\nu - 1}(z)\} </math> |
|
|
Abramowitz & Stegun (1995), p. 333, eq. (8.5.1) |
|||
|
NOTE: <math>~Q_\nu^\mu</math>, as well as <math>~P_\nu^\mu</math>, satisfies this same recurrence relation. |
Specifically, after adopting the association, <math>~\nu \rightarrow (n - \tfrac{1}{2})</math>, we have, when <math>~\mu = 0</math>,
|
<math>~Q_{n - \frac{1}{2}}^{1}(z)</math> |
<math>~=</math> |
<math>~ (n-\tfrac{1}{2}) (z^2-1)^{-\frac{1}{2}} [z Q_{n - \frac{1}{2}}(z) - Q_{n - \frac{3}{2}}(z)] </math> |
… |
for <math>~n \ge 1 \, ,</math> |
and, when <math>~\mu = 1</math>,
|
<math>~Q_{n - \frac{1}{2}}^{2}(z)</math> |
<math>~=</math> |
<math>~ (z^2-1)^{-\frac{1}{2}} \{ (n-\tfrac{3}{2}) z Q^1_{n - \frac{1}{2}}(z) - (n+\tfrac{1}{2})Q^1_{n - \frac{3}{2}}(z)\} </math> |
… |
for <math>~n \ge 1 \, .</math> |
As an example, the first of these two relations has been used to generate a column of numbers in the comparison table shown below for the toroidal function, <math>~Q^1_{+\frac{1}{2}}(z)</math>.
Comparison with Table IX from MF53
To facilitate copying & pasting for immediate use by other researchers, here we present in a tab-delimited, plain-text format the evaluation of nine separate toroidal functions: (Top half of table) <math>~P^0_{-\frac{1}{2}}</math>, <math>~P^0_{+\frac{1}{2}}</math> and <math>~P^0_{+\frac{3}{2}}</math>; (Bottom half of table) <math>~Q^0_{-\frac{1}{2}}</math>, <math>~Q^1_{-\frac{1}{2}}</math>, <math>~Q^2_{-\frac{1}{2}}</math>, <math>~Q^0_{+\frac{1}{2}}</math>, <math>~Q^1_{+\frac{1}{2}}</math> and <math>~Q^0_{+\frac{3}{2}}</math>. Each function has been evaluated for approximately 23 different argument values in the range, <math>~1.0 \le z \le 9.0</math>, and, for each function, two columns of function values have been presented: (Left column) Low-precision evaluation extracted directly from Table IX (p. 1923) of [MF53]; (Right column) Our double-precision evaluation based on a set of Numerical Recipes algorithms. One exception: The value listed under the "MF53" column for the evaluation of <math>~Q^0_{-\frac{1}{2}}(z=2.6)</math> is the high-precision value published on p. 340 of Abramowitz & Stegun (1995); notice that our high-precision evaluation matches all ten digits of their published value.
|
Top half of Table IX (p. 1923) of [MF53] |
z P0m1Half(z) P0p1Half(z) P0p3Half(z) MF53 Our Calc. MF53 Our Calc. MF53 Our Calc. 1.0 1.0000 1.0000 1.0000 1.2 0.9763 9.763155118E-01 1.0728 1.072784040E+00 1.3910 1.391015961E+00 1.4 0.9549 9.549467781E-01 1.1416 1.141585331E+00 1.8126 1.812643692E+00 1.6 0.9355 9.355074856E-01 1.2070 1.206963827E+00 2.2630 2.263020336E+00 1.8 0.9177 9.176991005E-01 1.2694 1.269362428E+00 2.7406 2.740570128E+00 2.0 0.9013 9.012862994E-01 1.3291 1.329138155E+00 3.2439 3.243939648E+00 2.2 0.8861 8.860804115E-01 1.3866 1.386583505E+00 3.7719 3.771951476E+00 2.4 0.8719 8.719279330E-01 1.4419 1.441941436E+00 4.3236 4.323569952E+00 2.6 0.8587 8.587023595E-01 1.4954 1.495416274E+00 4.8979 4.897875630E+00 2.8 0.8463 8.462982520E-01 1.5472 1.547181667E+00 5.4941 5.494045473E+00 3.0 0.8346 8.346268417E-01 1.5974 1.597386605E+00 6.1113 6.111337473E+00 3.5 0.8082 8.081851582E-01 1.7169 1.716877977E+00 7.7427 7.742702172E+00 4.0 0.7850 7.849616703E-01 1.8290 1.828992729E+00 9.4930 9.492973996E+00 4.5 0.7643 7.643076802E-01 1.9349 1.934919997E+00 11.3555 1.135475076E+01 5.0 0.7457 7.457491873E-01 2.0356 2.035563839E+00 13.3220 1.332184253E+01 5.5 0.7289 7.289297782E-01 2.1316 2.131629923E+00 15.3890 1.538897617E+01 6.0 0.7136 7.135750093E-01 2.2237 2.223681177E+00 17.5520 1.755159108E+01 6.5 0.6995 6.994692725E-01 2.3122 2.312174942E+00 19.8060 1.980569307E+01 7.0 0.6864 6.864402503E-01 2.3975 2.397488600E+00 22.1480 2.214774685E+01 7.5 0.6743 6.743481630E-01 2.4799 2.479937758E+00 24.5750 2.457459486E+01 8.0 0.6631 6.630781433E-01 2.5598 2.559789460E+00 27.0830 2.708339486E+01 8.5 6.525347093E-01 2.637271986E+00 2.967157094E+01 9.0 6.426376817E-01 2.712582261E+00 3.233677457E+01 |
|
Bottom half of Table IX (p. 1923) of [MF53] |
z Q0m1Half(z) Q1m1Half(z) Q2m1Half(z) Q0p1Half(z) Q1p1Half(z) Q0p3Half(z) MF53 Our Cal. MF53 Our Calc. MF53 Our Calc. M53 Our Calc. MF53 Our Calc. MF53 Our Calc. 1.1 2.8612 2.861192872E+00 2.3661 -2.366084077E+00 10.6440 1.064378304E+01 0.9788 9.787602829E-01 1.9471 -1.947110839E+00 0.4818 4.817841242E-01 1.2 2.5010 2.500956508E+00 1.7349 -1.734890983E+00 5.6518 5.651832631E+00 0.6996 6.995548314E-01 1.2524 -1.252395745E+00 0.2856 2.856355610E-01 1.4 2.1366 2.136571733E+00 1.2918 -1.291802851E+00 3.1575 3.157491205E+00 0.4598 4.597941602E-01 0.7618 -7.618218821E-01 0.14609 1.460918547E-01 1.6 1.9229 1.922920866E+00 1.0943 -1.094337965E+00 2.3230 2.323018870E+00 0.3430 3.430180260E-01 0.5501 -5.500770475E-01 0.09080 9.079816684E-02 1.8 1.7723 1.772268479E+00 0.9748 -9.748497733E-01 1.9018 1.901788930E+00 0.2720 2.720401772E-01 0.4285 -4.284853031E-01 0.06214 6.214026586E-02 2.0 1.6566 1.656638170E+00 0.8918 -8.917931374E-01 1.6454 1.645348489E+00 0.2240 2.240142929E-01 0.3489 -3.488955345E-01 0.04516 4.515872426E-02 2.2 1.5634 1.563378886E+00 0.8293 -8.292825549E-01 1.4712 1.471197798E+00 0.18932 1.893229696E-01 0.29263 -2.926294028E-01 0.03422 3.422108228E-02 2.4 1.4856 1.485653983E+00 0.7798 -7.797558474E-01 1.3441 1.344108936E+00 0.16312 1.631167365E-01 0.25076 -2.507568731E-01 0.02676 2.675556229E-02 2.6 1.419337751 1.419337751E+00 0.7391 -7.390875295E-01 1.2465 1.246521876E+00 0.14266 1.426580119E-01 0.21842 -2.184222751E-01 0.02143 2.143519083E-02 2.8 1.3617 1.361744950E+00 0.7048 -7.048053314E-01 1.1687 1.168702464E+00 0.12628 1.262756033E-01 0.19274 -1.927423405E-01 0.01751 1.751393553E-02 3.0 1.3110 1.311028777E+00 0.6753 -6.753219405E-01 1.1048 1.104816977E+00 0.11289 1.128885424E-01 0.17189 -1.718911443E-01 0.01454 1.454457729E-02 3.5 1.2064 1.206444997E+00 0.6163 -6.163068170E-01 0.9846 9.846190928E-01 0.08824 8.824567577E-02 0.13380 -1.338040913E-01 0.00966 9.664821286E-03 4.0 1.1242 1.124201960E+00 0.5713 -5.712994484E-01 0.8990 8.990205764E-01 0.07154 7.154134054E-02 0.10819 -1.081900595E-01 0.00682 6.819829619E-03 4.5 1.0572 1.057164923E+00 0.5353 -5.353494651E-01 0.8339 8.338659751E-01 0.05957 5.956966068E-02 0.08993 -8.992645608E-02 0.00503 5.029656514E-03 5.0 1.0011 1.001077380E+00 0.5057 -5.056928088E-01 0.7820 7.819717783E-01 0.05063 5.062950976E-02 0.07634 -7.633526879E-02 0.00384 3.837604899E-03 5.5 0.9532 9.532056775E-01 0.4806 -4.806378723E-01 0.7393 7.392682950E-01 0.04374 4.373774515E-02 0.06588 -6.588433822E-02 0.00301 3.008238619E-03 6.0 0.9117 9.116962715E-01 0.4591 -4.590784065E-01 0.7033 7.032568965E-01 0.03829 3.828867029E-02 0.05764 -5.763649873E-02 0.00241 2.410605139E-03 6.5 0.87524 8.752387206E-01 0.44025 -4.402537373E-01 0.67231 6.723067009E-01 0.03389 3.389003482E-02 0.05099 -5.098806037E-02 0.00197 1.967394932E-03 7.0 0.84288 8.428751774E-01 0.42362 -4.236198508E-01 0.64530 6.453008278E-01 0.03028 3.027740449E-02 0.04553 -4.553369214E-02 0.00163 1.630716095E-03 7.5 0.81389 8.138862008E-01 0.40877 -4.087751846E-01 0.62144 6.214442864E-01 0.02727 2.726650960E-02 0.04099 -4.099183107E-02 0.00137 1.369695722E-03 8.0 0.78772 7.877190099E-01 0.39542 -3.954155185E-01 0.60015 6.001530105E-01 0.02473 2.472532098E-02 0.03716 -3.716124286E-02 0.00116 1.163753807E-03 8.5 7.639406230E-01 -3.833053056E-01 5.809864341E-01 2.255696890E-02 -3.389458114E-02 9.987731857E-04 9.0 7.422062367E-01 -3.722587645E-01 5.636047532E-01 2.068890884E-02 -3.108168349E-02 8.648271474E-04 |
Relationships Between Various Associated Legendre Functions
|
Relationships Between Various Associated Legendre Functions |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
To insert a given equation into any Wiki document, type ... |
|
|||||||||||||||||
|
Template_Name |
Resulting Equation |
|||||||||||||||||
|
|
|||||||||||||||||
|
|
|||||||||||||||||
|
|
|||||||||||||||||
|
|
|||||||||||||||||
|
|
|||||||||||||||||
|
|
|||||||||||||||||
|
|
|||||||||||||||||
|
|
|||||||||||||||||
Key Parallel References (printed texts spanning 5+ decades)
- [C67] Chandrasekhar, S. 1967 (originally, 1939), An Introduction to the Study of Stellar Structure (New York: Dover)
- EQ_EOSideal0A — In C67, the ideal gas equation of state is initially written in terms of the specific volume <math>~V</math>, instead of the mass density <math>~\rho</math>; also, it is initially assumed that <math>~\bar{\mu}</math> = 1. Both <math>~\rho</math> and <math>~\bar{\mu}</math> are introduced in §III.1, Eq.(5).
- EQ_SSLaneEmden01 — At the end of his Chapter IV, C67 writes an extensive history of the earliest work on stellar structure pointing especially the origins of the so-called Lane-Emden equation. He points out, for example, that Ritter (1880) actually published this governing differential equation prior to Emden.
- [LL75] Laundau, L. D. & Lifshitz, E. M. 1975 (originally, 1959), Fluid Mechanics (New York: Pergamon Press)
- EQ_Continuity01 — LL75 present the Eulerian, rather than the Lagrangian form of the Continuity equation.
- EQ_Euler01 — In the Euler equation, LL75 do not initially include a source term to account for a gradient in the Newtonian gravitational potential, <math>~\Phi</math>; a term representing acceleration due to gravity, <math>\vec{g} = -\nabla\Phi</math>, is introduced in Eq.(2.4), but in LL75 this is intended primarily to describe gravity at the surface of the Earth.
- EQ_FirstLaw01 — LL75's Eq.(2.5) must be combined with their discussion of what they refer to as the familiar thermodynamic relation (between LL75 Eqs. 2.8 and 2.9) in order to appreciate the similarity with our expression.
- EQ_Poisson01 — In LL75, the symbol <math>\Delta</math>, rather than <math>\nabla^2</math>, is used to represent the Laplacian spatial operator.
- EQ_EOSideal0A — In LL75, the ideal gas equation of state is written in terms of the specific volume <math>~V</math>, as well as in terms of the mass density <math>~\rho</math>.
- [ST83] Shapiro, S. L. & Teukolsky, S. A. 1983, Black Holes, White Dwarfs, and Neutron Stars: The Physics of Compact Objects (New York: John Wiley & Sons); republished in 2004 by WILEY-VCH Verlag GmbH & Co. KGaA
- EQ_Continuity01 — ST83 present the Eulerian, rather than the Lagrangian form of the Continuity equation.
- [H87] Huang, K. 1987 (originally 1963), Statistical Mechanics (New York: John Wiley & Sons)
- EQ_Continuity01 — H87 presents the Eulerian, rather than the Lagrangian form of the Continuity equation, and the variable <math>\vec{u}</math> is used instead of <math>~\vec{v}</math> to represent the velocity.
- EQ_Euler01 — H87 presents the Eulerian, rather than the Lagrangian form of the Euler equation, and the variable <math>\vec{u}</math> is used instead of <math>~\vec{v}</math> to represent the velocity. Furthermore, to match the source term in our version of the Euler equation, we must set H87's applied acceleration, <math>\vec{F}/m = -\nabla</math><math>~\Phi</math>.
- EQ_FirstLaw01 — H87 begins a discussion of the 1st Law of Thermodynamics in the first section of the first chapter, but it does not appear in the form we present (relevant for a "dilute gas") until Eq.(4.31).
- [BT87] Binney, J. & Tremaine, S. 1987, Galactic Dynamics (Princeton, NJ: Princeton University Press)
- [KW94] Kippenhahn, R. & Weigert, A. 1994, Stellar Structure and Evolution (New York: Springer-Verlag)
- EQ_Continuity01 — KW94 present the Eulerian, rather than the Lagrangian form of the Continuity equation.
- EQ_FirstLaw01 — In KW94, the symbol <math>u</math> instead of <math>~\epsilon</math> is used to represent the specific internal energy.
- EQ_EOSideal0A — In KW94, the ideal gas equation of state is actually first introduced in §2.2, Eq.(27), but it is seriously discussed in Chapter 13. KW94 provide a particularly nice explanation of how to calculate the model parameter, <math>~\bar{\mu}</math>.
- EQ_SShydrostaticBalance01 — In KW94, the hydrostatic balance equation is expressed in terms of <math>dP/dM_r</math> instead of <math>dP/dr</math>; and the second term on the right-hand-side allows for a net radial acceleration.
- [HK94] Hansen, C. J. & Kawaler, S. D. 1994, Stellar Interiors: Physical Principles, Structure, and Evolution (New York: Springer)
- [P00] Padmanabhan, T. 2000, Theoretical Astrophysics. Volume I: Astrophysical Processes (Cambridge: Cambridge University Press); and Padmanabhan, T. 2001, Theoretical Astrophysics. Volume II: Stars and Stellar Systems (Cambridge: Cambridge University Press)
- EQ_Poisson01 — See also Vol.I: §10.4, Eq.(10.58).
- [BLRY07] Bodenheimer, P., Laughlin, G. P., Różyczka, M. & Yorke, H. W. 2007, Numerical Methods in Astrophysics An Introduction (New York: Taylor & Francis)
Other Equations with Assigned Templates
|
To insert a given equation into any Wiki document, type ... |
|||||||||||||||
|
Template_Name |
Resulting Equation |
Description |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
<math>~\frac{\partial\rho}{\partial t} + \nabla \cdot (\rho \vec{v}) = 0</math> |
Eulerian (and Conservative) form of the continuity equation. |
||||||||||||||
|
<math>~\frac{\partial\vec{v}}{\partial t} + (\vec{v}\cdot \nabla) \vec{v}= - \frac{1}{\rho} \nabla P - \nabla \Phi</math> |
Eulerian form of the Euler equation. |
||||||||||||||
|
<math>~\frac{\partial(\rho\vec{v})}{\partial t} + \nabla\cdot [(\rho\vec{v})\vec{v}]= - \nabla P - \rho \nabla \Phi</math> |
Conservative form of the Euler equation. |
||||||||||||||
|
<math>~\frac{\partial\vec{v}}{\partial t} + \vec\zeta \times \vec{v}= - \frac{1}{\rho} \nabla P - \nabla \biggl[\Phi + \frac{1}{2}v^2 \biggr] </math> |
Euler equation in terms of vorticity. |
||||||||||||||
|
<math>~\frac{d\epsilon}{dt} + P \frac{d}{dt} \biggl(\frac{1}{\rho}\biggr) = 0</math> |
Adiabatic form of the 1st Law of Thermodynamics. |
||||||||||||||
|
<math>~P = K_\mathrm{n} \rho^{1+1/n}</math> |
Polytropic equation of state. |
||||||||||||||
|
<math>~H = (n+1)K_\mathrm{n} \rho^{1/n}</math> |
Enthalpy in a polytrope. |
||||||||||||||
|
<math>~\rho = \biggl[ \frac{H}{(n+1)K_\mathrm{n}} \biggr]^n </math> |
Density in terms of enthalpy for polytrope. |
||||||||||||||
|
<math>~P = n_g k T</math> |
Alternate form of the ideal gas equation of state. |
||||||||||||||
|
<math>~P = (\gamma_\mathrm{g} - 1)\epsilon \rho </math> |
Alternate form of the ideal gas equation of state. |
||||||||||||||
|
Gravitational potential exterior to an axisymmetric torus, |
||||||||||||||
|
Gravitational potential of any axisymmetric mass distribution. |
||||||||||||||

|
|---|
|
© 2014 - 2021 by Joel E. Tohline |